Menu
Analysis of α-S8 crystal structure.
Algorithm:
1. Open the database S8_P4_Cl2 and compute an adjacency matrix for the first record.
2. Run the program IsoCryst.
3. Be sure that in Options/Bonds tab only valence bonds are checked in the Take column.
4. Grow the structure to get the whole molecules.
5. Select a molecule and show only the selected one.
6. Check Specific and van der Waals boxes in the Show and Take columns.
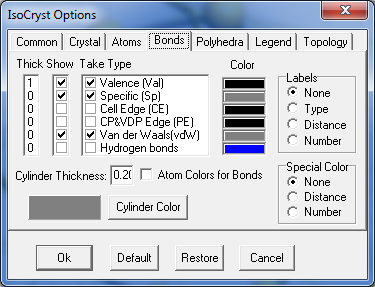
7. Perform growth one time only to get all atoms connected with the selected molecule by non-valence interactions.

8. Uncheck Specific and van der Waals boxes, unselect the molecule and perform growth a few more times to get all surrounding molecules.
9. Select all molecules (click outside the molecules, or use the mouse with the tools

 and find total number of selected atoms (128) in the status line.
and find total number of selected atoms (128) in the status line.

So there are 128/8=16 molecules in the image and, hence, the central molecule is surrounded by 15 other molecules (the molecular coordination number is 15)
Exercise: compute molecular coordination numbers for phosphorus P4 (56/4=14) and chlorine Cl2 (28/2=14) molecules (see the database S8_P4_Cl2).
Questions: Examine the differences between Specific and Van der Waals contacts looking at the distances values in the Adjacency Matrix of chlorine Cl2. Using only vdW contacts the neighbor molecules are 10, and 4 using only Spec. This indicate that there are 4 halogen bonds at work. By default, ToposPro detects halogen bonds and classify them as Specific bonds. For more on analysis of molecular packing see:
Peresypkina E.V., Blatov V.A. Molecular coordination numbers in crystal structures of organic compounds. Acta Cryst. 2000, B56, 501-511.
Peresypkina E.V., Blatov V.A. Topology of molecular packings in organic crystals. Acta Cryst. 2000, B56, 1035-1045.
For the definition of the halogen bond see: Desiraju G.R., Ho P.S., Kloo L., Legon A.C., Marquardt R., Metrangolo P., Politzer P., Resnati G., Rissanen K. Definition of the halogen bond (IUPAC Recommendations 2013) Pure Appl. Chem. 2013, 85, 1711-1713
Algorithm:
1. Open the database S8_P4_Cl2 and compute an adjacency matrix for the first record.
2. Run the program IsoCryst.
3. Be sure that in Options/Bonds tab only valence bonds are checked in the Take column.
4. Grow the structure to get the whole molecules.
5. Select a molecule and show only the selected one.
6. Check Specific and van der Waals boxes in the Show and Take columns.

7. Perform growth one time only to get all atoms connected with the selected molecule by non-valence interactions.

8. Uncheck Specific and van der Waals boxes, unselect the molecule and perform growth a few more times to get all surrounding molecules.
9. Select all molecules (click outside the molecules, or use the mouse with the tools

So there are 128/8=16 molecules in the image and, hence, the central molecule is surrounded by 15 other molecules (the molecular coordination number is 15)
Exercise: compute molecular coordination numbers for phosphorus P4 (56/4=14) and chlorine Cl2 (28/2=14) molecules (see the database S8_P4_Cl2).
Questions: Examine the differences between Specific and Van der Waals contacts looking at the distances values in the Adjacency Matrix of chlorine Cl2. Using only vdW contacts the neighbor molecules are 10, and 4 using only Spec. This indicate that there are 4 halogen bonds at work. By default, ToposPro detects halogen bonds and classify them as Specific bonds. For more on analysis of molecular packing see:
Peresypkina E.V., Blatov V.A. Molecular coordination numbers in crystal structures of organic compounds. Acta Cryst. 2000, B56, 501-511.
Peresypkina E.V., Blatov V.A. Topology of molecular packings in organic crystals. Acta Cryst. 2000, B56, 1035-1045.
For the definition of the halogen bond see: Desiraju G.R., Ho P.S., Kloo L., Legon A.C., Marquardt R., Metrangolo P., Politzer P., Resnati G., Rissanen K. Definition of the halogen bond (IUPAC Recommendations 2013) Pure Appl. Chem. 2013, 85, 1711-1713